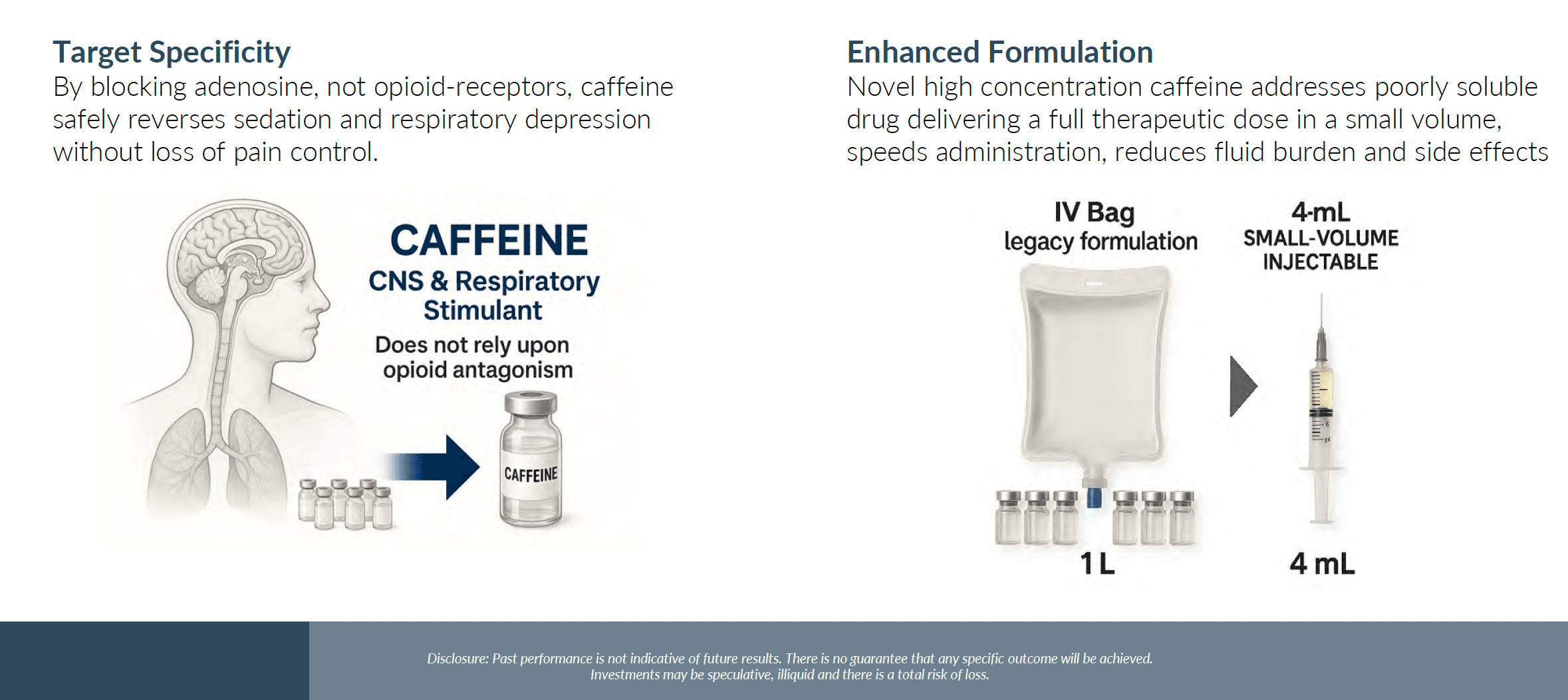
OYE Therapeutics, a clinical-stage pharmaceutical company, announced it raised $5.475 million in a Series A financing on November 28, 2025. The funding will advance development of the company's proprietary high-concentration caffeine therapy for anesthesia recovery and opioid-induced respiratory depression (OIRD). The company expects to begin a Phase 1 clinical trial in the first quarter of 2026.
The importance of this development lies in addressing two critical healthcare challenges: improving recovery from anesthesia and combating the opioid overdose crisis. The investigational therapy represents a novel approach to these problems, particularly for OIRD where it is designed to be used alongside naloxone to support wakefulness and respiratory drive during recovery. This dual application could have significant implications for both surgical settings and emergency overdose response.
OYE Therapeutics is pursuing a broad platform that includes military and civilian acute care applications, building on preclinical work and U.S. military grant funding. The company's approach focuses on transforming clinical problems that have been traditionally accepted as intractable into solvable challenges. The therapy's mechanism is distinct from naloxone, potentially offering complementary benefits in overdose situations.
The financing was oversubscribed, indicating strong investor interest in alternative approaches to the opioid crisis and anesthesia management. WBB Securities LLC acted as the placement agent for the transaction. For more information about the company's approach, visit https://www.oyetherapeutics.com. Additional details about the financial aspects can be found through WBB Securities at https://www.wbbsec.com.
This development matters because it represents a potential advancement in two areas of significant public health concern. For patients undergoing anesthesia, improved recovery protocols could mean shorter hospital stays and reduced complications. For communities affected by the opioid epidemic, an additional tool to combat overdoses could save lives, particularly as fentanyl-related intoxications continue to rise nationally. The military applications also suggest potential benefits for battlefield medicine and emergency response scenarios.
The clinical trial timeline indicates that meaningful data about the therapy's safety and efficacy could become available within the next few years. If successful, this approach could eventually provide healthcare providers with new options for managing post-anesthesia recovery and supporting patients through opioid overdose recovery. The funding announcement signals progress toward addressing these persistent healthcare challenges through innovative pharmaceutical development.